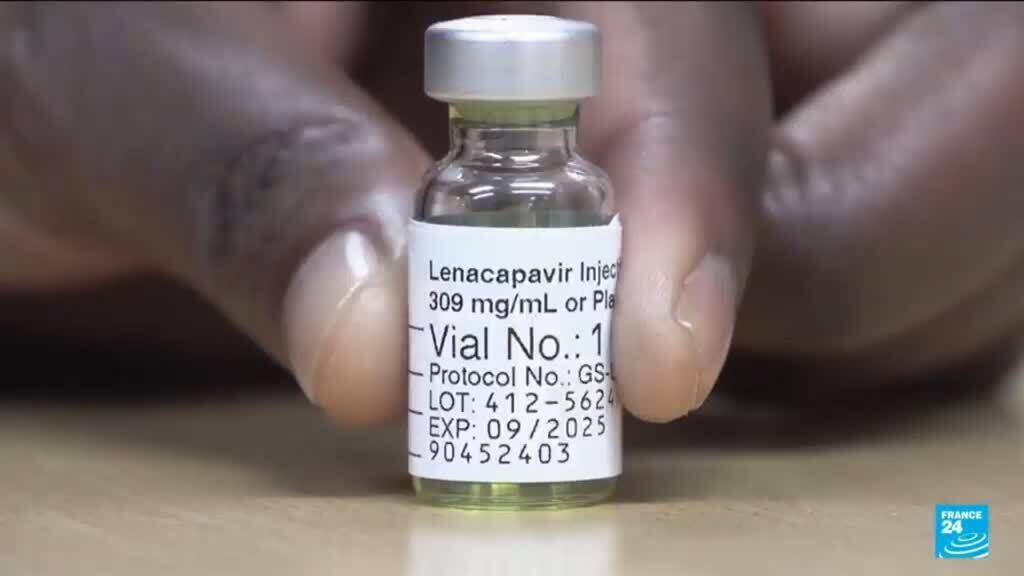The Us Food and Drug Administration On Wednesday Approved Gilead Sciences Lenacapavir, A Twice-Yearly Injvirection, for Preventing Hiv Infection in Adults and Adolescents at High Risk of Contracting the Deadly Virus. Lenacapavir Proveed Nearly 100% Effective at Preventing Hiv in Large Trials Last Year, Raising New Hope of Interrupting Transmission of The Virus That Infects 1.3 Million People A Year.
Source link
Us Promise Promising Professional HIV Treatment